NEWS
Mpox Outbreak: Nigeria Receives 10,000 JYNNEOS® Vaccines from U.S.
In a significant development for Nigeria’s public health efforts, the country has received a donation of 10,000 JYNNEOS® vaccines from the United States. This donation marks a crucial step in addressing the ongoing Mpox outbreak, particularly in high-risk areas across the nation.
During the donation ceremony in Abuja, U.S. Ambassador to Nigeria, Richard Mills, announced that the JYNNEOS® vaccines, which are specifically approved for the prevention of Mpox, will be distributed nationwide. The distribution will focus on regions with the highest reported cases of the disease. This initiative is part of a broader global effort to curb the spread of Mpox and strengthen health security worldwide.
Read Also:
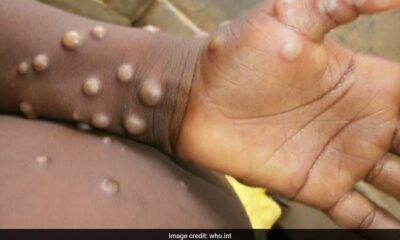
NCDC confirms 62 cases of Monkeypox in the country
Monkeypox: Entire British Airways crew in isolation after one tests positive
Mpox, formerly known as Monkeypox, has been a persistent challenge in Nigeria. The current outbreak has intensified the urgency for effective public health responses. Mpox, a zoonotic disease transmitted from animals to humans and spread through close human contact, has necessitated enhanced surveillance and intervention efforts.
Ambassador Mills emphasized the importance of this donation amidst rising global Mpox cases. “This generous donation of JYNNEOS Mpox vaccines underscores the strong partnership between Nigeria and the United States in advancing public health. These vaccines are essential in protecting our population, especially in high-risk areas where Mpox poses a significant threat,” he stated.
The U.S. government’s support is in line with global health organizations’ efforts, including the World Health Organization (WHO) and Nigeria’s National Centre for Disease Control (NCDC), to contain outbreaks and prevent further spread of Mpox. Mills reiterated the commitment of the U.S. to collaborate with Nigeria in controlling the disease and safeguarding public health.
Dr. Walter Kazadi Mulombo, WHO Country Representative to Nigeria, highlighted the importance of a comprehensive approach to health risk management. “We must ensure that treatment, diagnosis, and post-vaccine care are accessible to all affected populations while continuously improving our health strategies,” he said. Mulombo expressed gratitude to the U.S. for its support and praised Nigeria’s proactive measures in advancing health initiatives.
Ms. Christian Munduate, UNICEF Representative in Nigeria, commended the U.S. for its unwavering support. “The solidarity and support from the U.S. Government, especially through these vaccines, is truly remarkable and deeply appreciated by the people of Nigeria,” she remarked. Munduate stressed the critical role of primary health care in responding to outbreaks and the importance of strengthening local healthcare systems to protect vulnerable populations.
In his address, Prof. Ali Mohammed Pate, Coordinating Minister of Health and Social Welfare, expressed deep gratitude for the international community’s support. Represented by Ms. Daju Kachollom, Permanent Secretary of the Ministry of Health, Pate praised the contributions of development partners like USAID, WHO, and UNICEF. “Their support is vital in addressing Nigeria’s health needs effectively,” he said.
Dr. Muyi Aina, Executive Director of the National Primary Health Care Development Agency (NPHCDA), announced that the vaccines will be administered to healthcare workers, frontline responders, and high-risk populations. States with the highest Mpox burden will receive prioritized distribution to effectively curb the virus’s spread. Aina urged the public to adhere to preventive measures and remain vigilant.
For further information or to report suspected cases of Mpox, the public can contact the NCDC via the following:
NCDC Toll-Free Number: 6232
SMS: 08099555577
WhatsApp: 07087110839
Website: www.ncdc.gov.ng
JYNNEOS®, also known as Imvamune® or Imvanex® internationally, is a third-generation vaccine licensed for the prevention of smallpox and Mpox. Recommended by the Advisory Committee on Immunization Practices (ACIP) for those at risk of orthopoxvirus infections, JYNNEOS® has been a key tool in the U.S. response to the Mpox outbreak since 2022. The vaccine, based on the Modified Vaccinia Ankara (MVA) virus, does not replicate efficiently in humans and is fully licensed for use in adults aged 18 and older. It became commercially available in the U.S. on April 1, 2024.